Influenza A/B/H1N1 Antigen Rapid Test

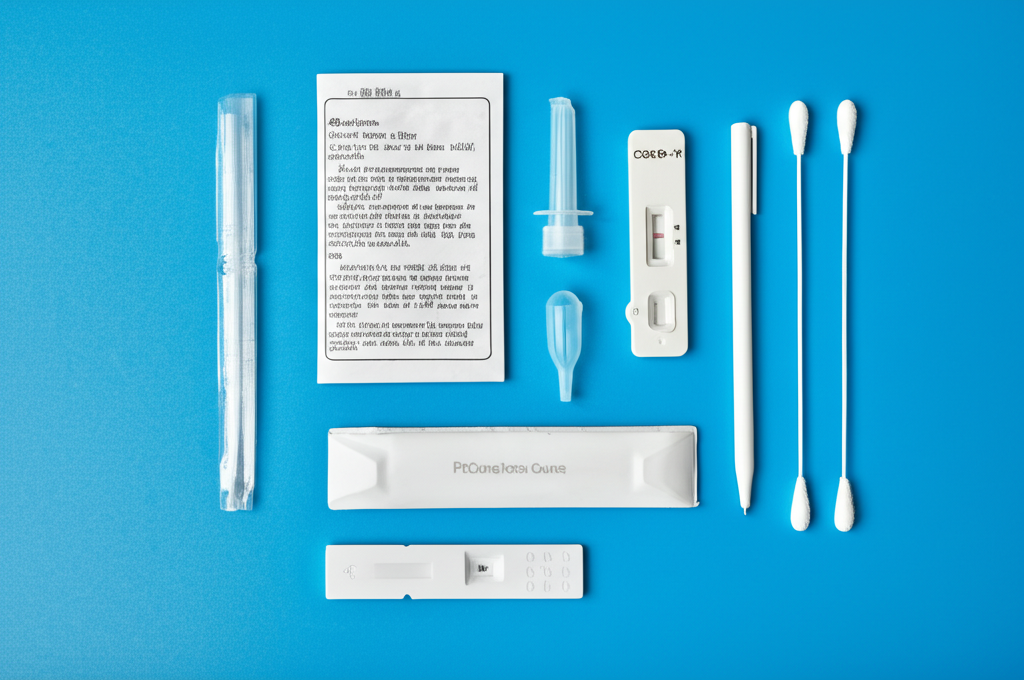
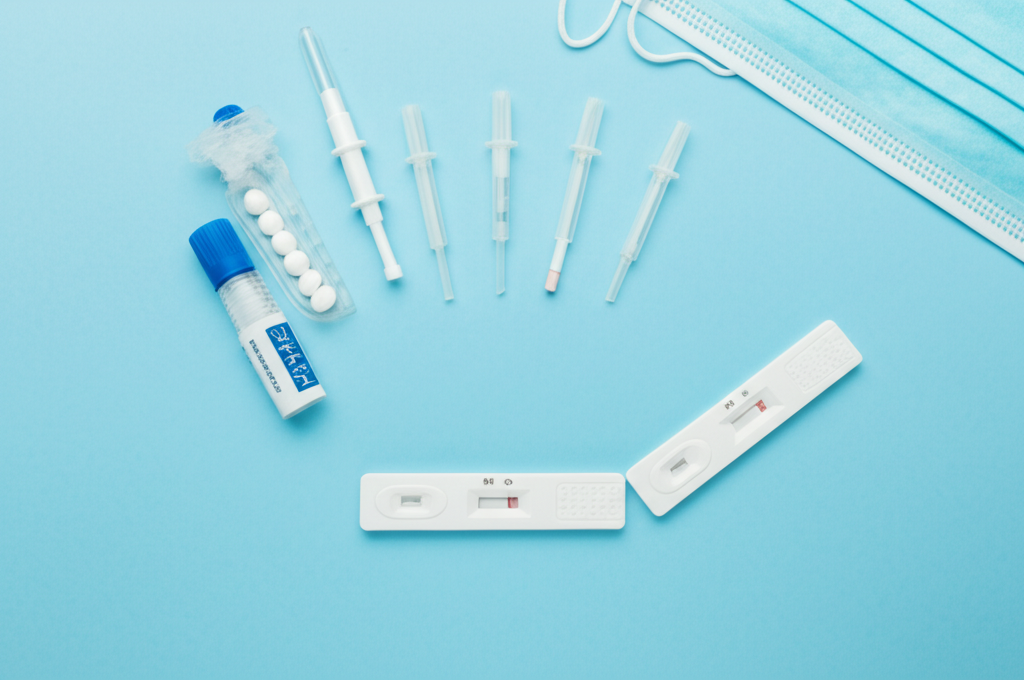
Product Description
The Influenza A/B/H1N1 Antigen Rapid Test is a qualitative in vitro diagnostic device for the rapid detection of influenza A and B, including the H1N1 subtype, viral antigens directly from nasopharyngeal swab specimens. This test provides results in minutes, aiding in the timely diagnosis and management of influenza infections. The test employs an immunochromatographic assay, allowing healthcare professionals to quickly identify infected individuals and initiate appropriate treatment or infection control measures. Early detection helps reduce the spread of influenza, minimizes hospitalizations, and improves patient outcomes. The test is designed for use in various settings, including doctor's offices, clinics, and emergency rooms, providing rapid point-of-care results.
Features
-
Rapid results (within 15 minutes)
-
Easy-to-use cassette format
-
High sensitivity and specificity
Specifications
| Parameter | Specification |
|---|---|
| Detection Target | Influenza A/B/H1N1 antigens |
| Sample Type | Nasopharyngeal swab |
| Methodology | Immunochromatography |
| Detection Range | Positive/Negative |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30°C |
| Shelf Life | 18 months |
Product Advantages
Early Screening Tool
Helps quickly identify infected individuals, implement timely isolation measures, and control the spread of the epidemic.
Suitable for Multiple Scenarios
Suitable for various scenarios such as home self-testing, corporate screening, schools, and clinics.
Reliable Quality
Strict quality control system ensures stable and reliable product performance.
Instructions for Use
- Read the instructions carefully.
- Prepare sample collection tools and the test kit.
- Collect the sample according to the instructions.
- Add the sample to the test cassette.
- Wait 15 minutes to read the results.
Certification Information
- CE Certified
- ISO13485
Interested in Influenza A/B/H1N1 Antigen Rapid Test?
Contact us for pricing, bulk orders, or any questions you may have.
